Over 10 years we help companies reach their financial and branding goals. Engitech is a values-driven technology agency dedicated.
411 University St, Seattle, USA
engitech@oceanthemes.net
+1 -800-456-478-23
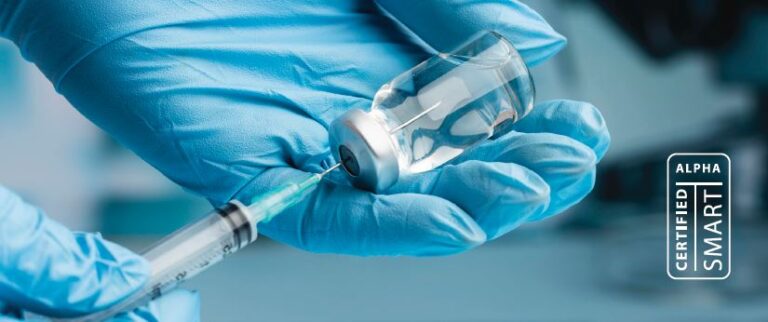
In this critical application, a pharmaceutical sterile injectable supplier reached out with a material issue causing end product failures. In this case, an LSR (liquid silicone resin) was used as a vial cap.
The same manufacturer of a different drug delivery product was having issues with their polymer-based delivery system not injection molding properly. It was determined that the same raw material (PM) for the LSR was being purchased from the same supplier but produced at different facilities providing the nomenclature.
Download the case study to learn more about how a Premier ESR from Alpha Technologies helped solve these issues for these manufacturers.